SARS-COV-2 Neutralizing Antibody Test Kit(LFIA) 1PCS/BOX
The SARS-CoV-2 Neutralizing antibody Test Kit(LFIA) is suitable for in vitro qualitative detection of SARS-CoV-2 neutralizing antibodies in human serum, plasma, or whole blood samples (capillary or venous) including samples prepared by commonly-used anticoagulants (K2EDTA, Na Citrate, Li-Heparin) from individuals with vaccine injection people and recovered people.
Retail price
$ 0
Market price
$ 0
Category:
重量
0
Stock:
0
隐藏域元素占位
- Product Detail
- Accessories
- Technical Specification
- Product Tags
-
Intended Application
The SARS-CoV-2 Neutralizing antibody Test Kit(LFIA) is suitable for in vitro qualitative detection of SARS-CoV-2 neutralizing antibodies in human serum, plasma, or whole blood samples (capillary or venous) including samples prepared by commonly-used anticoagulants (K2EDTA, Na Citrate, Li-Heparin) from individuals with vaccine injection people and recovered people. SARS-CoV-2 Neutralizing antibody can bind to the pathogenic target proteins RBD and NTD on the surface of the virus, thereby preventing the virus from binding to cell surface receptors. The development of a vaccine depends on whether the neutralizing antibody is produced by immunization, so the detection of the neutralizing antibody is crucial to assess the effectiveness of the vaccine. SARS-CoV-2 Neutralizing antibody Test Kit(LFIA) can quickly and accurately detect neutralizing antibodies, which is of great signifcance for the development of COVID- 19 vaccines, evaluation of effectiveness, and evaluation of neutralizing antibody levels in the population.
Content of a Kit
One test kit contains: Test Cassettes |1 Buffer Solution Bottle | 1 Package Insert
Type I test cassette contains
● A test strip in a plastic cassette
● Dried reagents with stabilizers
● Colloidal gold-labeled recombinant SARS-CoV-2 protein NTD
● Colloidal gold-labeled mouse lIgG
● Goat anti-mouse polyclonal antibody
● Mouse anti-human lgG monoclonal antibodyType II test cassette contains
● A test strip in a plastic cassette
● Dried reagents with stabilizers
● Colloidal gold-labeled recombinant SARS-CoV-2 protein RBD
● Colloidal gold-labeled mouse lgG
● Goat anti-mouse lgG polyclonal antibody
● cMouse anti-human IgG monoclonal antibodyType III test cassette contains
● A test strip in a plastic cassette
● Dried reagents with stabilizers
● Colloidal gold-labeled recombinant SARS-CoV-2 protein NTD
● Colloidal gold-labeled recombinant SARS-CoV-2 protein RBD
● Colloidal gold-labeled mouse lgG
● Goat anti-mouse polyclonal antibody
● Mouse anti-human IgG monoclonal antibodyWarnings and Precautions
● For human in vitro clinical diagnostics only.
● The product should only be used by healthcare professionals or trained technicians.
● After opening the sealed cassette pouch the test should be used within one hour.
● Do not immerse test cassette in water
● Do not freeze test cassette or buffer solution.
● Handle specimens in accordance to the OSHA Standard on Bloodborne Pathogens.
● Wear protective gloves, clothing, and eyewear.
● Wash hands thoroughly after handling specimens.
● Dispose of all used or damaged test cassettes, buffer solution bottle or other kit components as biohazardous materials.
● Do not use test cassette, buffer solution, or any other kit components if the pouch is damaged orthe seal is broken.
● Do not use samples containing lipids, hemolysis, or turbidity which can affect results.
● This package insert must be read completely before performing the test. Failure to follow directionsin package insert may yield inaccurate test results.
● Test results should be read bet ween 15 and 20 rninutes after s specimen is ap plied to the sammiple well. Results read after 20 minutes may give erroneous results.
● Do not use test cassette, buffer solution, or any kit component beyond the indicated expiration
date.
● Bring all reagents to room temperature before LIse.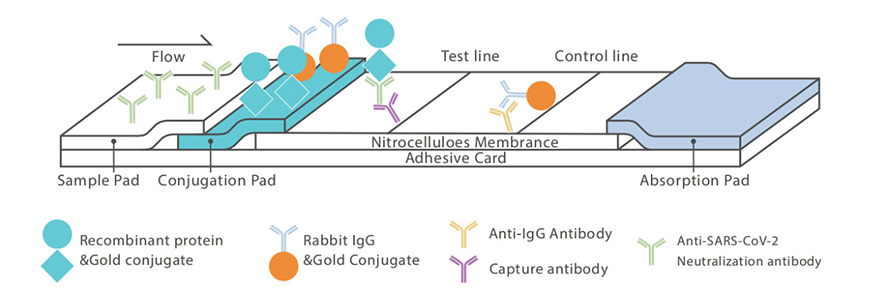
Previous











